WHAT AM I DOING TO HELP KIDS ACHIEVE?
HOW DO I KNOW WHEN THEY ARE THERE?
WHAT IS THE EVIDENCE?
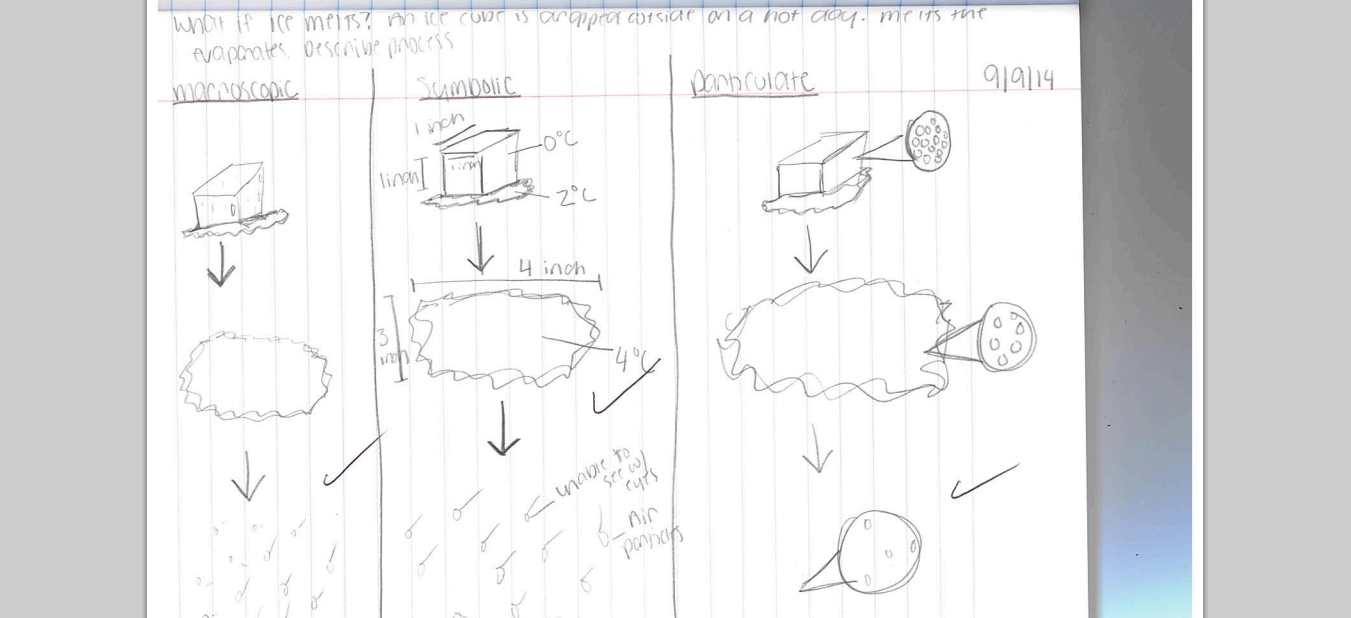
So I decided to try the TIMU project that teacher's helped me develop this summer. It involved just introducing the particulate level with the macroscopic and symbolic. As a quick "assessment", I had students story board something as simple as ice melting and then the water evaporating. For the most part, they kind of got the idea (see above). However...if you look at the water phase on the particulate level drawing you will notice it looks more like a gas than a liquid with one free surface. Almost every student did this. I gave them credit for demonstrating some type of change but I also know it is not correct. We have not gone into phase changes that much on the particulate level so I felt this was a fair misconception on the students part. The following day, we started off class with a couple of particulate drawings of water and we addressed this misconception. I might follow up with a test question as well to see if they were paying attention.
This week we are back to the symbolic and macroscopic world of measuring. In one class they will be measuring aluminum by taking several measurements of beads and cylinders and trying to determine which method is the best with the least amount of error. In another class I am going to (got this from Mike Geyer and Kim Stites) take an exact amount of water measured from a buret and have students measure it in graduated cylinders and large beakers and then ask, which devices provide the most accurate and precise measurements? I will let you know how it goes.....
No comments:
Post a Comment